Coccidiosis
Etiology:
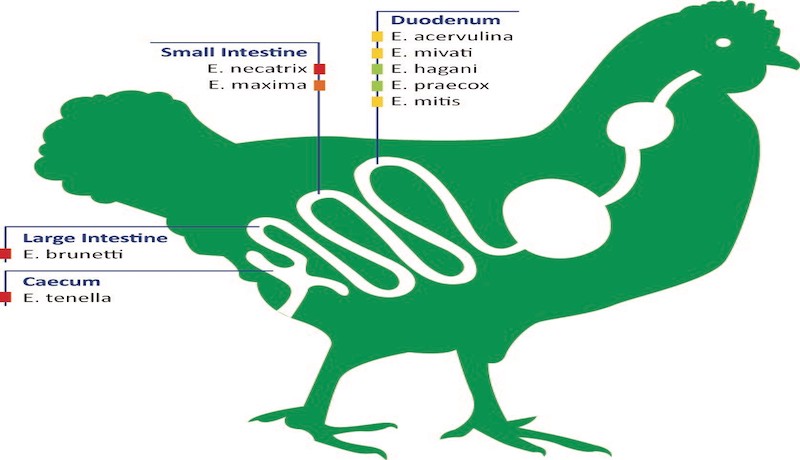
Various Eimeria spp. that parasitize specific portions of the intestinal tract of chickens.
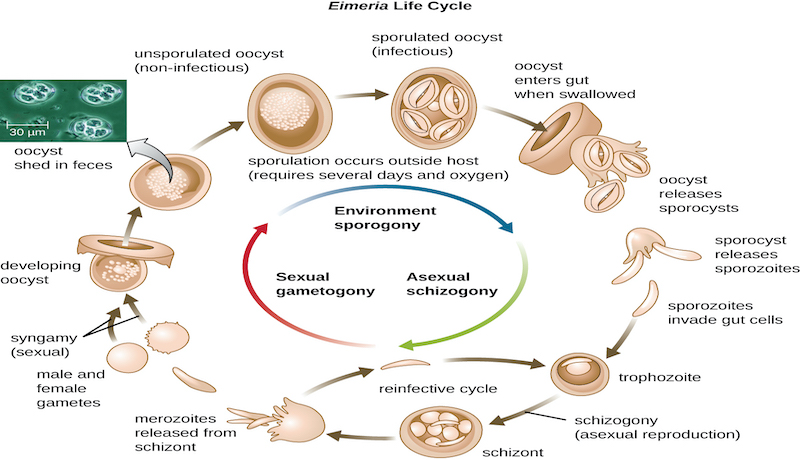
Coccidia Life Cycle
Occurrence and Economic Significance:
Coccidiosis occurs world-wide and is a major cause of mortality and suboptimal growth and feed conversion efficiency in immature flocks unless appropriate preventive measures are implemented. The cost of anticoccidial feed additives and treatment is estimated to exceed $400 million annually in all poultry producing areas of the world.
Transmission:
The sporulated oocyst is the infective stage of the life-cycle. Infected, recovered chickens shed oocysts representing a problem in multi-age operations. Oocysts can be transmitted mechanically on the clothing and footwear of personnel, contaminated equipment, or in some cases, by wind spreading poultry-house dust and litter over short distances.
Factors contributing to outbreaks of clinical coccidiosis include:
– litter moisture content exceeding 30% due to the ingress of rain or leaking waterers.
– immunosuppression (Marek’s disease, IBD, mycotoxins)
– suboptimal inclusion of anticoccidials or incomplete distribution (poor mixing) in the feed.
– environmental and managemental stress such as overstocking, inoperative feeding systems, inadequate ventilation.
Clinical Signs:
Administration of amprolium solution, 0.024% of the active ingredient in drinking water for 3 – 5 days. Sulfonamides (sulfamethazines , 0.1% for 2 days, 0.05% for 4 days or commercial combinations of sulfa drugs) in drinking water. Administration of water dispersable vitamin A and K supplements may enhance recovery.
Lesions:
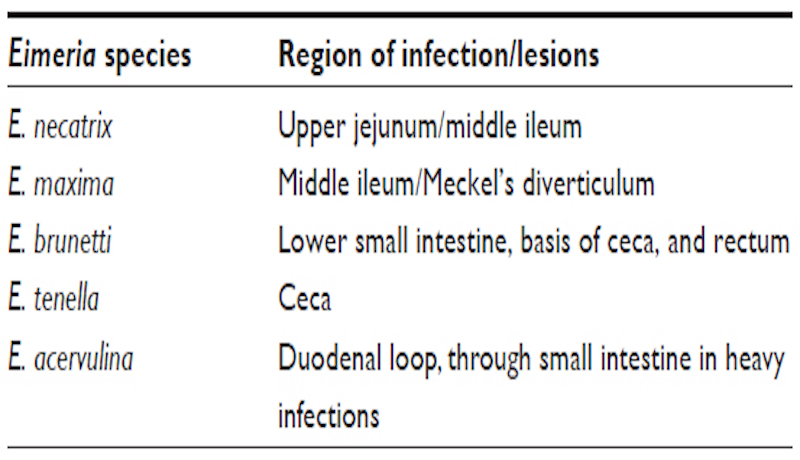
– E. acervulina and E. mivati: 1-2mm areas of hemorrhage interspersed with white foci visible through the serosa of the distal duodenum and proximal jejunum.
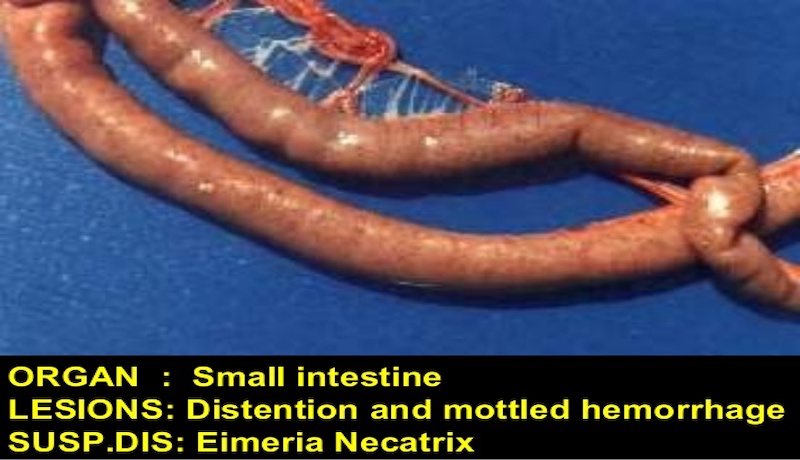
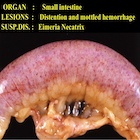
– E. necatrix: severe distention of the mid-jejunum with hemorrhages in the mucosa and red-stained fluid in the lumen.
– E. maxima: distention of the mid-jejunum with hemorrhages in the mucosa.
– E. tenella: hemorrhagic typhlitis (inflammation of the cecum).
– E. brunetti: hemorrhages of the mucosa of the distal jejunum and colon. Fibrinonecrotic enteritis may occur in chronic cases.
To confirm a diagnosis in a commercial operation the following specimens should be submitted to a laboratory:
– Intestine from a sacrificed, affected bird preserved in 5% potassium dichromate for culture and identification of Eimeria sp.
– Intestine showing gross lesions in 10% formalin for histological examination.
– Representative feed samples for anticoccidial assay.
– Litter samples for oocyst counts.
Treatment:
– Administration of RoyalProl Forte 500 gm / 900 liter of drinking water. For 5-7 days.
– Administration of water-dispersible vitamin A and K supplements may enhance recovery.
Prevention:
Anticoccidial vaccines are appropriate for replacement breeding stock and roasters. This approach is cost-effective but requires experienced and diligent management and monitoring especially if the vaccine is applied overfeed. Intraocular administration by spray or the insertion of a gelatine cylinder impregnated with oocysts in the chick delivery box contributes to an even distribution of vaccine through the flock.
Management procedures that limit saturation of litter include:
– Appropriate installation and management of water systems. Nipple drinkers reduce spillage of water onto litter compared to bell and trough drinkers.
– Acceptable ventilation rate.
– Maintaining recommended stocking density.
– Providing adequate feeding space.
– Inclusion of anticoccidials in diets at recommended levels will prevent clinical infection.
– Chemical and ionophore anticoccidials for broilers in shuttle programs.
– Synthetic coccidiostats for breeders and floor-reared commercial egg production flocks.